Everyday I Have the Oxide Blues
Description
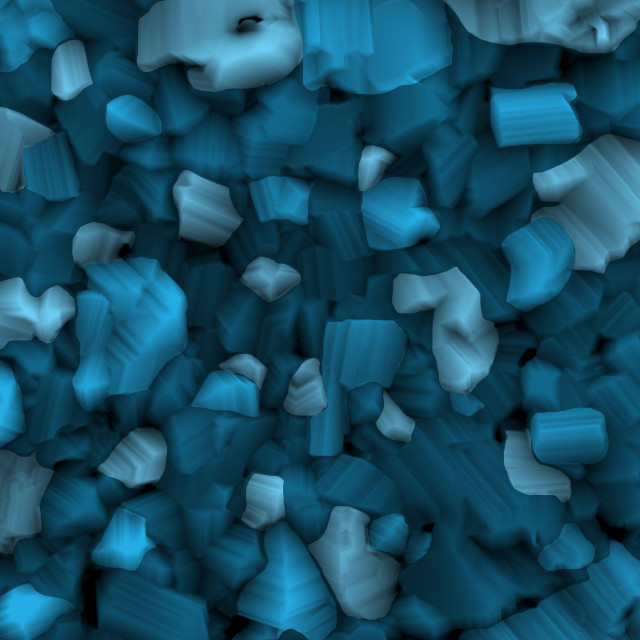
Titanium metal is extremely reactive to oxygen. When it comes into contact with oxygen, abundantly present in the atmosphere, it undergoes a chemical reaction known as oxidation. In this reaction, oxygen strips off electrons from titanium, similar to the actions of a paint-stripper. The reaction results in the production of various substances known as titanium oxides, some of which are dominant over others. However, during the process, some of the oxides do not form. The oxides that are formed have the blues from missing all of their fellow oxides that did not form.
This image was taken using a scanning electron microscope. This microscope uses a beam of electrons which reflect off the surface of the oxides to form an image. The oxides shown, were formed after nine hours of oxidation at 800 degrees C in a gettered argon environment with very low oxygen content. In a gettered environment, reactive material is placed and maintained inside a vacuum system. The color was added to different grains solely for aesthetic purposes and does not correspond to any specific condition or grain orientation.